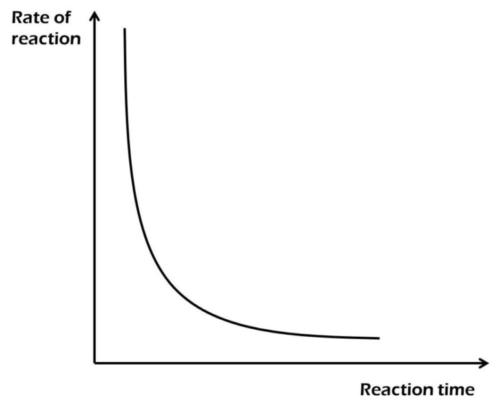
The diagram above best illustrates the effect of decrease in
concentration
temperature
surface area
pressure
Correct answer is A
No explanation has been provided for this answer.
The condition required for corrosion to take place is the presence of
water and carbon (IV) oxide
water, carbon (IV) oxide and oxygen
oxygen and carbon (IV) oxide
water and oxygen
Correct answer is D
No explanation has been provided for this answer.
catalyst
temperature
concentration
light
Correct answer is D
No explanation has been provided for this answer.
The function of zinc electrode in a galvanic cells is that it
undergoes reduction
serves as the positive electrode
production of electons
uses up electrons
Correct answer is C
If an external electrical conductor connects the copper and zinc electrodes, zinc from the zinc electrode dissolves into the solution as Zn2+ ions (oxidation), releasing electrons that enter the external conductor.
A chemical reaction which the hydration energy is greater than the lattice energy is referred to as
a spontaneous reaction
an endothermic reaction
an exothermic reaction
a reversible reaction
Correct answer is C
When the hydration energy is greater than the lattice energy, then it is exothermic.
JAMB Subjects
Aptitude Tests